Back to Courses







Life Sciences Courses - Page 13
Showing results 121-130 of 644

Health Information Technology Fundamentals
In this course you will receive an overview of the health IT ecosystem with a specific focus on the role of electronic health records (EHRs). You’ll be introduced to the factors that contributed to the move from paper records to digitized records and who the most common vendors are. We’ll go over features of EHRs such as computerized provider order entry, clinical decision support, documentation capabilities, and medication reconciliation. Like a physician’s stethoscope, the EHR has become an important tool in healthcare delivery and plays a part throughout the patient’s journey. You’ll go through each of the steps from patient scheduling, to front desk registration, outpatient visits, emergency room encounters, and inpatient admissions.
During the course, we’ll also cover examples of how technical issues related to the EHR can be as simple as problems with logging or password resets. But how they can also be more complex related to alerts that are firing and the display of information. Although some of those challenges are beyond the scope of the IT support staff, having familiarity with the scope of potential problems and the broader EHR landscape is important. This course also includes an introduction to database architecture, servers, and interfaces. We wrap up by discussing the importance of training end-users on healthcare technology and the way in which effective change management strategies are crucial.

Introduction to Breast Cancer
Welcome to an Introduction to Breast Cancer! In this course, we’ll learn a bit about the leading cause of cancer in women worldwide – from the basic biology of the disease, to risk factors and prevention, to treatment modalities to survivorship. We’ll talk to leading experts, explore some of the milestone studies that have pushed this field forward, and have interactive discussions on discussion boards and social media. You’ll even have an opportunity to let us know what topics you want to cover on tweetchats, so we can try to make the content fit your interests.
There is something in this course for everyone – if you’re a breast cancer survivor or the friend/family member of someone with this disease, this course will help you to better understand this disease, and give you ideas for questions you may want to ask your doctor. Maybe you’re a healthcare provider or studying to be the same, this course is a great refresher on where the state of the science is. If you’re a healthcare administrator wondering about how the interdisciplinary components of breast cancer care fit together, or an entrepreneur thinking about unmet needs in this space, or someone in public health interested in prevention, this course is also for you!
Are you ready to learn a lot, and have some fun while we’re at it? If so, I hope you’ll join us! Let’s get started!!!

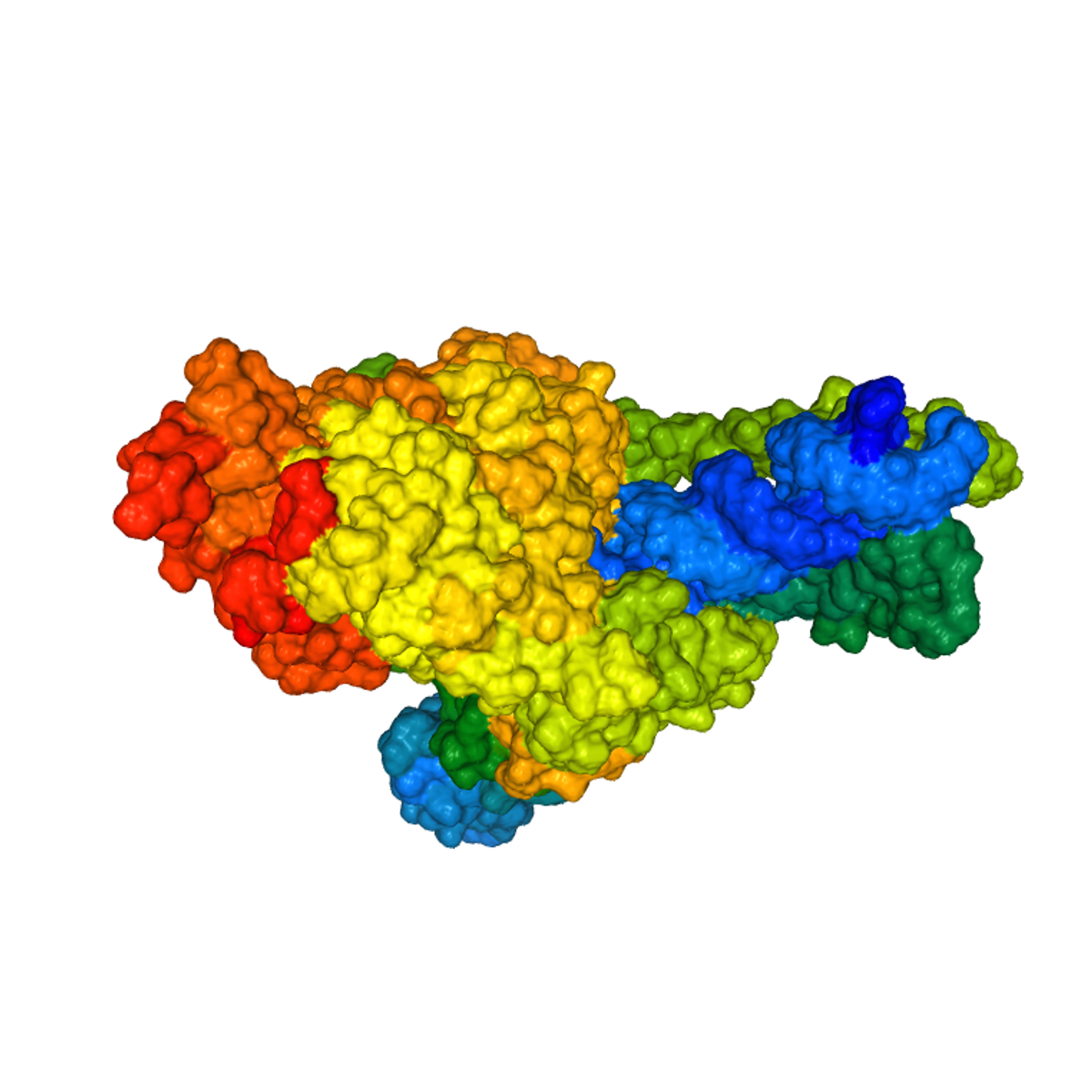
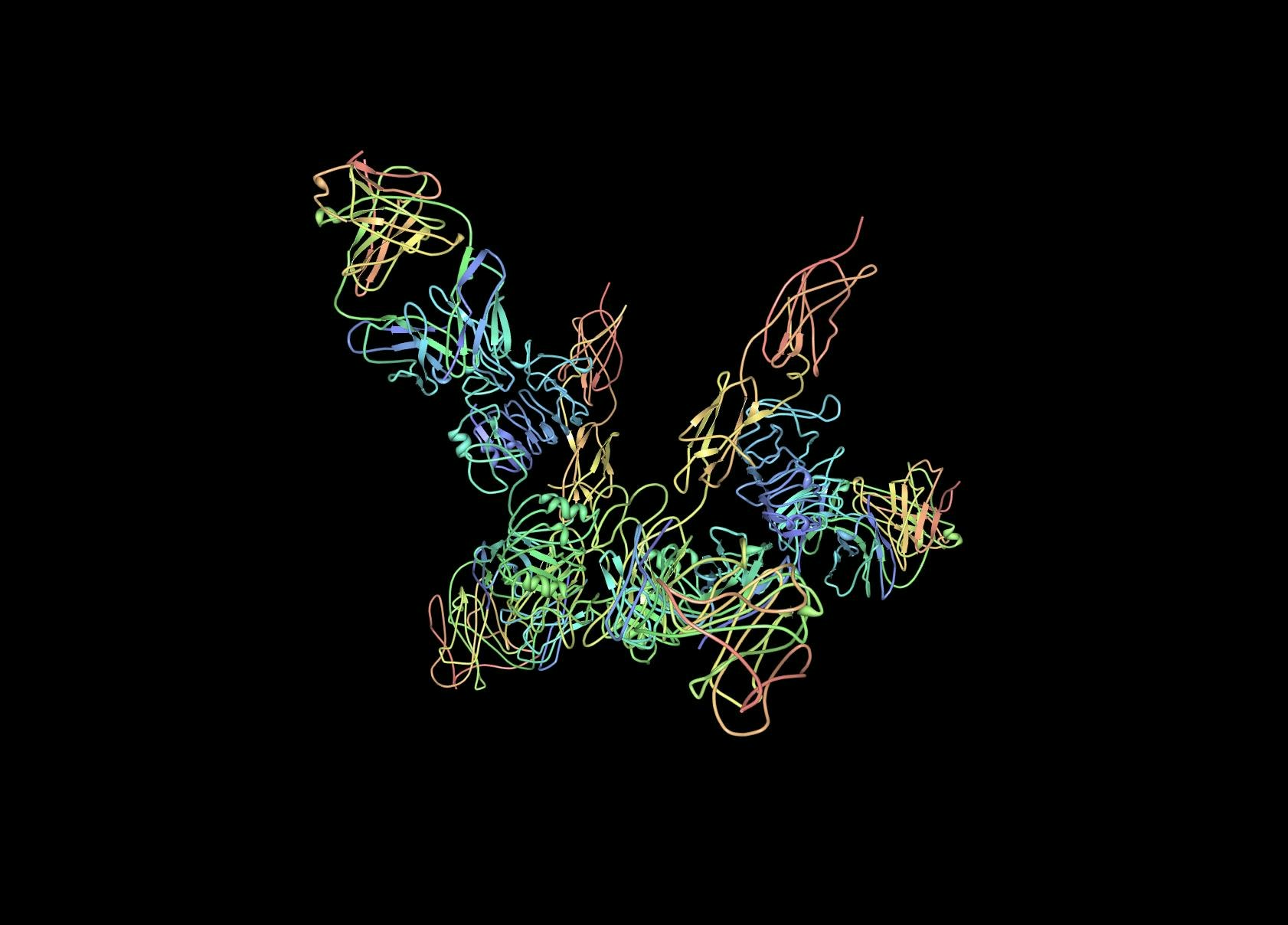
3D SARS-CoV-19 Protein Visualization With Biopython
In this project you will create an interactive three-dimensional (3D) representation of SARS-CoV-19 (Coronavirus) protein structures & publication-quality pictures of the same, understand properties of SARS-CoV-19 genome, handle biological sequence data stored in FASTA & PDB (Protein Data Bank) and XML format, and get insights from this data using Biopython. This hands-on project will also give you a glimpse of tasks a Bioinformatician performs on a daily basis, along with the up-to-date concepts and database use cases in the field of Medical Research and Human genetics.
In this project, we will also cover basics about important databases used by biologists and biotechnologists, along with the type of sequence data we can access and visualize from these databases using Biopython & Jupyter notebook.

Introduction to Participatory Approaches in Public Health
This course will introduce you to participatory approaches to public health. You will learn about the history of participatory health research and why it is essential to solving contemporary public health challenges. The course will help you to understand the social and cultural context of public health, before introducing you to essential concepts for working with communities: knowledge and power. Finally, you will engage with critical analyses of participatory approaches, to help you to determine if and when such strategies are appropriate. Throughout the course you will analyse real-world case studies of community-based health projects, including historical HIV social movements, public health projects with sex workers, and participatory approaches to the COVID-19 pandemic. The course will equip you to practice public health in partnership with local communities. It is followed by a second course, Applying Participatory Approaches in Public Health Settings, which builds upon the theoretical foundations of this introductory course.

Finding Hidden Messages in DNA (Bioinformatics I)
Named a top 50 MOOC of all time by Class Central!
This course begins a series of classes illustrating the power of computing in modern biology. Please join us on the frontier of bioinformatics to look for hidden messages in DNA without ever needing to put on a lab coat.
In the first half of the course, we investigate DNA replication, and ask the question, where in the genome does DNA replication begin? We will see that we can answer this question for many bacteria using only some straightforward algorithms to look for hidden messages in the genome.
In the second half of the course, we examine a different biological question, when we ask which DNA patterns play the role of molecular clocks. The cells in your body manage to maintain a circadian rhythm, but how is this achieved on the level of DNA? Once again, we will see that by knowing which hidden messages to look for, we can start to understand the amazingly complex language of DNA. Perhaps surprisingly, we will apply randomized algorithms, which roll dice and flip coins in order to solve problems.
Finally, you will get your hands dirty and apply existing software tools to find recurring biological motifs within genes that are responsible for helping Mycobacterium tuberculosis go "dormant" within a host for many years before causing an active infection.

Lecture Series for Preventing and Controlling COVID-19
In December 2019, a cluster of patients with pneumonia of unknown cause appeared in Wuhan, Hubei. Governments at all levels and Department of Health Administration highly valued and rapidly organized the centers for Disease Control and Prevention (CDC), medical institutions, and scientific research academies to conduct a survey, rescue, and collaborative research. This new antigen was quickly identified as a novel coronavirus, named as 2019-nCoV by World Health Organization (WHO). Meanwhile, the pneumonia caused by it was named as novel coronavirus pneumonia. Implementing the decisions by the CPC Central Committee and State Council with Xi at the core, the whole country took comprehensive actions in social mobilization and deployment, taking epidemic control a most important work in the immediate future. The spread of the epidemic has been effectively controlled. However, novel coronavirus has spread to the whole world thereafter, so it called for closer international cooperation in epidemic prevention and control. Based on this situation, it was urgent to set a new MOOC program about COVID-19 and impart scientific knowledge of the disease to foreign students, undergraduate students, and all the other common people abroad, laying a solid foundation for the triumph of the war against COVID-19.
The novel coronavirus is a new strain of coronavirus that had never been found in humans before. This huge family already known can cause cold or Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS) and other severe diseases. The main signs and symptoms of the infected people include respiratory symptoms, fever, dry cough, shortness of breath, and dyspnea, etc. In severe and critical cases, there will appear pneumonia, severe acute respiratory syndrome, kidney failure, and even death. The two-month war against COVID-19 has helped us accumulate loads of experience in disease prevention, control, diagnosis, and treatment, which can be shared with the whole world as a reference.
In front of the need of our students and common people on COVID-19 prevention knowledge, the Health Science Center of Xi’an Jiaotong University has organized related experts to issue the English lectures of the epidemic prevention and control for people at home and abroad and international community. The contents are mainly about “Introduction,Diagnose,therapeutic Strategy and Prevention for COVID-19”. Through this course, we sincerely hope all our students and the common peoples of the world have scientific knowledge of COVID-19 and increased self-protection awareness, improving international community’s capacity for the prevention and control of COVID-19. We should work in great unity, take science-based and targeted measures against the epidemic, and have confidence in conquering the virus.

Evidence-based Toxicology
Welcome to the Evidence-based Toxicology (EBT) course. In medicine and healthcare, evidence-based medicine has revolutionized the way that information is evaluated transparently and objectively. Over the past ten years, a movement in North America and Europe has attempted to translate this revolution to the field of toxicology.
The Center for Alternatives to Animal Testing (CAAT) within the department of Environmental Health and Engineering at the Johns Hopkins Bloomberg School of Public Health hosts the first chair for EBT and the secretariat for the EBT Collaboration on both sides of the Atlantic. Based on the Cochrane Collaboration in Evidence-based Medicine, the EBT Collaboration was established at the CAAT to foster the development of a process for quality assurance of new toxicity tests for the assessment of safety in humans and the environment.
Regulatory safety sciences have undergone remarkably little change in the past fifty years. At the same time, our knowledge in the life sciences is doubling about every seven years. Systematic review and related evidence-based approaches are beginning to be adapted by regulatory agencies like the Environment Protection Agency (EPA), the European Food Safety Authority (EFSA), and the US National Toxicology Program. They provide transparent, objective, and consistent tools to identify, select, appraise, and extract evidence across studies.
This course will showcase these emerging efforts and address opportunities and challenges to the expanded use of these tools within toxicology.
Case Studies in Personalized Medicine
Learn how advances in biomedicine hold the potential to revolutionize drug development, drug treatments, and disease prevention: where are we now, and what does the future hold? This course will present short primers in genetics and mechanisms underlying variability in drug responses. A series of case studies will be used to illustrate principles of how genetics are being brought to bear on refining diagnoses and on personalizing treatment in rare and common diseases. The ethical and operational issues around how to implement large scale genomic sequencing in clinical practice will be addressed.
After completing this course, learners will understand
1. The ways in which genetic variants can contribute to human disease susceptibility
2. How to choose among drug therapies based on genetic factors
3. That the functional consequences of the vast majority of genetic variants discovered by modern sequencing are unknown.
This course is targeted primarily at physicians 5+ years out of training. Other healthcare providers, medical/health sciences students, and members of the public may also be interested.
Course launches January 15, 2016.
* The information presented in “Case Studies in Personalized Medicine” is offered for educational and informational purposes only, and should not be construed as personal medical advice. If you have questions or concerns about a medical matter, please consult your doctor or other professional healthcare provider.

Drug Development
The University of California San Diego, Skaggs School of Pharmacy and Pharmaceutical Sciences Drug Development course brings you lectures from both faculty and industry experts. With this course, recorded on campus at UCSD, we seek to share our access to top people in the field who bring an unprecedented range of expertise on drug development.
In this course you will learn the different stages of clinical development as well as the regulatory including but not limited to, an Investigational New Drug Application (IND), New Drug Application (NDA), and product labeling. Additionally you will learn how to Incorporate study design methods for consideration in the design of clinical protocols to assess safety, tolerability, and efficacy in multiple therapeutic areas.
In this course you will learn the different phases of clinical development:
* Phase 1 or early stage clinical trial are conducted primarily to determine how the new drug works in humans, its safety profile and to predict its dosage range. It typically involves between 30 and 100 healthy volunteers.
* Phase 2 or Proof of Concept POC studies test for efficacy as well as safety and side effects in a group of between 30 to 200 hundred patients with the disease for which the new drug is being developed.
* Phase 3 or late stage clinical development involve much larger group of patients, between a few hundred to thousands, depending on the indication, which will help determine if the new drug can be considered both safe and effective. It will involve control groups using placebo and/or current treatment as a comparison.
* Product registration and approval process after a drug is considered safe and effective from Phase 3 trials, it must be authorized in each individual country before it can be marketed. All data generated about the small molecule or biologic is collected and submitted to the regulatory authorities in the US at the FDA, Food and Drug Administration FDA, in Europe the EMA or European Medicines Agency, Japan Ministry of Health and other countries which may require their own national approvals.
This course is intended as part 2 of a series: Drug Discovery (https://www.coursera.org/learn/drug-discovery), Drug Development and Drug Commercialization (https://www.coursera.org/learn/drug-commercialization). We would highly recommend that you take the courses in order since it will give you a better understanding on how a drug is discovered in the lab before being tested in clinical trials and then launched in the market place.

One Health: Pandemic preparedness, prevention, and response
This course was developed by the Karolinska Institutet (KI) and the Federation of the European Academies of Medicine (FEAM) as part of the research project 'Pan-European Response to the Impacts of the COVID-19 and future Pandemics and Epidemics' (PERISCOPE). Funded by the European Commission Research Funding programme Horizon 2020 under the Grant Agreement number 101016233, PERISCOPE investigates the broad socio-economic and behavioural impacts of the COVID-19 pandemic, to make Europe more resilient and prepared for future large-scale risks.
This course is primarily aimed at highly specialised technical professional groups (healthcare authorities, policymakers, researchers and other academics) interested in learning more about the One Health approach. The modules are for participants who are likely to have previous knowledge about the concept in one specific area or pillar but not necessarily in all of them.
The course will provide basic knowledge and contextualisation of One Health in creeping crises, such as the COVID-19 pandemic. Attending the course, participants will identify enablers, limitations, barriers, and next steps in and to the One Health concept and operationalisation.
PERISCOPE website: https://www.periscopeproject.eu/
KI website: https://ki.se/en
FEAM website: https://www.feam.eu/
Popular Internships and Jobs by Categories
Find Jobs & Internships
Browse
© 2024 BoostGrad | All rights reserved