Back to Courses





Chemistry Courses - Page 3
Showing results 21-30 of 31
General Chemistry: Concept Development and Application
This course will cover the topics of a full year, two semester General Chemistry course. We will use a free on-line textbook, Concept Development Studies in Chemistry, available via Rice’s Connexions project.
The fundamental concepts in the course will be introduced via the Concept Development Approach developed at Rice University. In this approach, we will develop the concepts you need to know from experimental observations and scientific reasoning rather than simply telling you the concepts and then asking you to simply memorize or apply them.
So why use this approach?
One reason is that most of us are inductive learners, meaning that we like to make specific observations and then generalize from there. Many of the most significant concepts in Chemistry are counter-intuitive. When we see where those concepts come from, we can more readily accept them, explain them, and apply them.
A second reason is that scientific reasoning in general and Chemistry reasoning in particular are inductive processes. This Concept Development approach illustrates those reasoning processes.
A third reason is that this is simply more interesting! The structure and reactions of matter are fascinating puzzles to be solved by observation and reasoning. It is more fun intellectually when we can solve those puzzles together, rather than simply have the answers to the riddles revealed at the outset.
Recommended Background:
The class can be taken by someone with no prior experience in chemistry. However, some prior familiarity with the basics of chemistry is desirable as we will cover some elements only briefly. For example, a prior high school chemistry class would be helpful.
Suggested Readings:
Readings will be assigned from the on-line textbook “Concept Development Studies in Chemistry”, available via Rice’s Connexions project. In addition, we will suggest readings from any of the standard textbooks in General Chemistry. A particularly good free on-line resource is Dickerson, Gray, and Haight, "Chemical Principles, 3rd Edition". Links to these two texts will be available in the Introduction module.

Fundamentals of Macroscopic and Microscopic Thermodynamics
Course 1 first explores the basics of both macroscopic and microscopic thermodynamics from a postulatory point of view. In this view, the meaning of temperature, thermodynamic pressure and chemical potential are especially clear and easy to understand. In addition , the development of the Fundamental Relation and its various transformations leads to a clear path to property relations and to the concept of ensembles needed to understand the relationship between atomic and molecular structural properties and macroscopic properties. We then explore the relationship between atomic and molecular structure and macroscopic properties by taking a statistical point of view. Using a postulatory approach, the method for doing this is made clear. This leads to the development of the partition function which describes the distribution of molecular quantum states as a function of the independent, macroscopic thermodynamic properties.

Dense Gases, Liquids and Solids
Course 4 of Statistical Thermodynamics addresses dense gases, liquids, and solids. As the density of a gas is increased, intermolecular forces begin to affect behavior. For small departures from ideal gas behavior, known as the dense gas limit, one can estimate the change in properties using the concept of a configuration integral, a modification to the partition function. This leads to the development of equations of state that are expansions in density from the ideal gas limit. Inter molecular potential energy functions are introduced and it is explored how they impact P-V-T behavior. As the density is increased, there is a transition to the liquid state. We explore whether this transition is smooth or abrupt by examining the stability of a thermodynamic system to small perturbations. We then present a brief discussion regarding the determination of the thermodynamic properties of liquids using concept of the radial distribution function (RDF), and how the function relates to thermodynamic properties. Finally, we explore two simple models of crystalline solids.

Non-Equilibrium Applications of Statistical Thermodynamics
Course 5 of Statistical Thermodynamics explores three different applications of non-equilibrium statistical thermodynamics.
The first is the transport behavior of ideal gases, with some discussion of transport in dense gases and liquids. It starts with simple estimates of the transport properties of an ideas gas. It then introduces the Boltzmann Equation and describes the Chapman-Enskog solution of that equation in order to obtain the transport properties. It closes with a discussion of practical sources of transport properties.
Spectroscopic methods have become increasingly common as a way of determining the thermodynamic state of a system. Here we present the underlying concepts of the subject and explores how spectroscopy can be used to determine thermodynamic and flow properties.
Chemical kinetics are important in a variety of fluid/thermal applications including combustion, air quality, fuel cells and material processing. Here we cover the basics of chemical kinetics, with a particular focus on combustion. It starts with some definitions, including reaction rate and reaction rate constant. It then explores methods for determining reaction rate constants. Next, systems of reactions, or reaction mechanisms, are explored, including the oxidation of hydrogen and hydrocarbon fuels. Finally, computational tools for carrying out kinetic calculations are explored.

Chemistry
This course is designed to cover subjects in advanced high school chemistry courses, correlating to the standard topics as established by the American Chemical Society. This course is a precursor to the Advanced Chemistry Coursera course. Areas that are covered include atomic structure, periodic trends, compounds, reactions and stoichiometry, bonding, and thermochemistry.
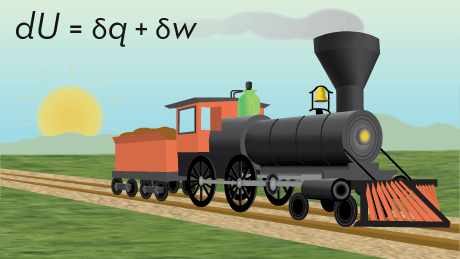
Statistical Molecular Thermodynamics
This introductory physical chemistry course examines the connections between molecular properties and the behavior of macroscopic chemical systems.

Teaching in University Science Laboratories (Developing Best Practice)
This course is developed to improve the effectiveness of laboratory classes in higher education. It aims to support teachers to improve their teaching skills for active learning in university science laboratory courses. It will show you how laboratory sessions can differ with respect to their aim and expected learning outcomes, how to engage students for learning and how to cope with their different levels of pre-knowledge and experience and probe their understanding. Last but not least it will show how you could assess students in laboratory courses.
This course is developed by ECTN (European Chemistry Thematic Network), Working group Lecturing Qualifications and Innovative Teaching Methods.

The Science of Gastronomy
This course introduces several basic scientific principles underpinning the methodology of cooking, food preparation, and the enjoyment of food. All topics covered have a strong basis in biology, chemistry, and physics application. Among others, they include the consumption of cooked food, the physiological and evolutionary implications of the senses, geographic and cultural influences on food, and the rationale behind food preparation. We will also discuss issues such as coupling of senses to improve sense stimulation; altering flavor by chemical means, and modification of the coloration to improve the appearance of dishes. Following the video demonstrations of the scientific principles of cooking, you will learn to recognize the key ingredients and their combinations for preparing good healthy food. You will also be asked to try out and practice specific cooking principles through the weekly assignments; analyze your data and make comparisons of your experiences with others.
At the end of this course, you will be able to:
- appreciate the scientific basis of various recipes.
- develop your own recipes by integrating some of the scientific principles into new dishes.
- recognize the influence of the material world on human perception from the different senses.
- appreciate the art of integrating science into cooking and dining.
Important Note: This course is not designed for people with special dietary needs such as vegetarian, diabetic, and gluten-free diets. If you feel uncomfortable with any part of the assignments or activities of this course, you can substitute some of the ingredients or ask friends and family members to help with the tasting of your assignments. Alternatively, you may skip that specific assignment if you have fulfilled all other qualifying requirements to pass the course.
Course Overview video: https://www.coursera.org/lecture/gastronomy/course-overview-43gyz

Exploring Quantum Physics
An introduction to quantum physics with emphasis on topics at the frontiers of research, and developing understanding through exercise.

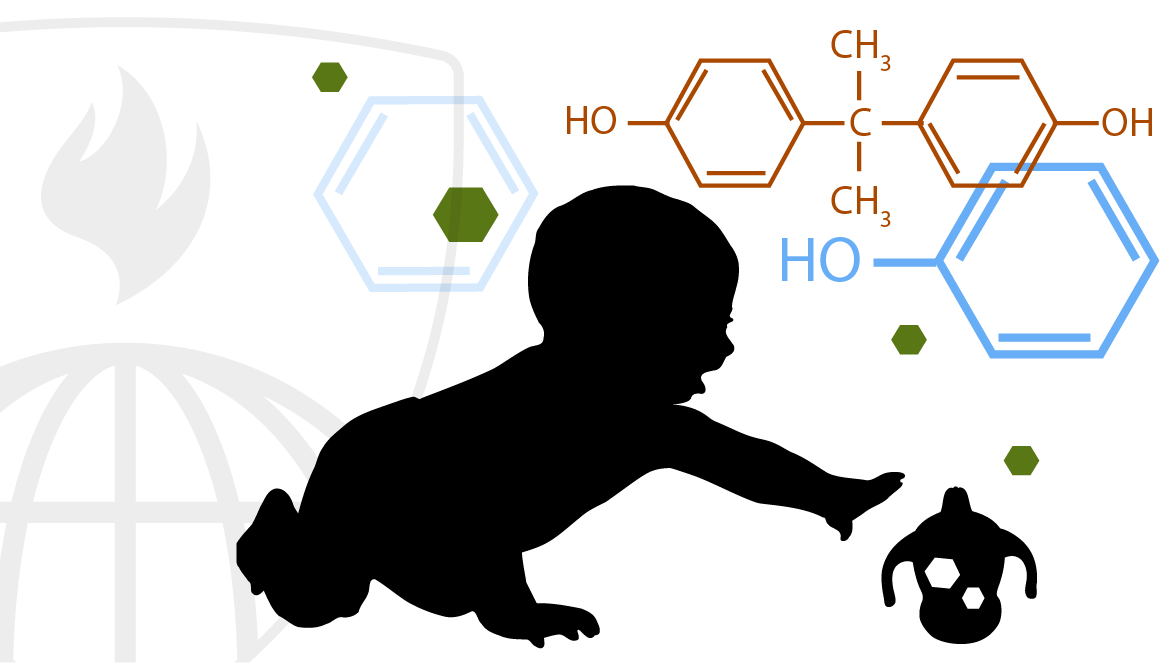
Chemicals and Health
This course covers chemicals in our environment and in our bodies and how they impact our health. It addresses policies and practices related to chemicals, particularly related to how they get into our bodies (exposures), what they do when they get there (toxicology), how we measure them (biomonitoring) and their impact on our health. Most examples are drawn from the US.
Popular Internships and Jobs by Categories
Find Jobs & Internships
Browse
© 2024 BoostGrad | All rights reserved